Quality is at the heart of manufacturing companies’ profitability. Quality drives revenues with its impact on brand image or customer satisfaction. Quality also determines costs, since preventing defects, or catching them earlier and more reliably, results in lower costs per unit. Quality can also determine success with regulatory compliance, accountability and speed-to-market—some of today’s most pressing issues. A breakthrough in quality can accelerate business success.
If quality isn’t a hot topic or is viewed as old news, there is usually one of two reasons. The first reason: The company took on total quality management (TQM) in the 1980s and now it’s an ingrained part of its culture. Managers systematically use data to drive out waste, variability and defects in all their business processes. Their disciplined quality methodologies help them succeed with Lean, Six Sigma and other initiatives.
Or, as the second reason, the company has not adopted a rigorous approach to quality. These companies often don’t make steady improvements. If they do, they often don’t tie quality gains to business results, so management is not interested, and does not believe a quality methodology will help them remain competitive.
The 20 manufacturers interviewed for this article illustrate a variety of paths to, and benefits from, quality breakthroughs. These paths vary from business process improvements, to process- and documentation-oriented quality management systems, to enterprise resource planning (ERP) quality modules, to plantwide operations software, to test systems, to process automation improvements and combinations of these. The companies represented use products from AssurX, Camstar, CEBOS, EMNS, Eyelit, Freedom, iBaseT, IBS Americas, Lynk, Peoplesoft, Rockwell Automation, SAP, Tecnomatix and TIP Technology. No matter the path toward improved quality or system used, these manufacturers’ issues and keys to success can accelerate quality gains.
Technology and process changes
Quality programs can result in staggering improvements. Some are achieved almost instantly upon making a change, while others occur over a period of months or years by following a rigorous process for continuous improvement. Figure 1 shows some examples.
There is great variety in areas and percentages of benefit among the companies interviewed for this article. However, they agree that their breakthroughs were the result of a change in both processes and automated systems to support them. One comment was, “Without a quality process, technology will not solve any problem.”
Technology plays a number of roles in quality improvement. Much of this is consistent, whether the technology is process or test automation, statistical process control (SPC), manufacturing execution systems (MES), corrective and preventative action (CAPA), quality assurance (QA), documentation management and non-conformance workflow, laboratory information management systems (LIMS) or ERP.
Improve data integrity:People inadvertently introduce errors in manually entering data. One respondent’s research showed that only 92 percent of re-keyed data is entered correctly.
Aid efficiency: If a system takes on the routine tasks, people can focus keeping the process running and on solving the most significant problems.
Speed the process: Automatically assembling product history and certification documentation prevents delays in production and delivery. Data analysis and proactive action is much faster when data is electronic, not in paper files or separate documents.
Improve analysis: While people are constantly analyzing the data they have, technology can correlate and analyze more data more consistently, faster and at less cost.
Proof point: A system can accurately record improvements and correlate them to benefits. “Technology and automation has helped link quality and cost savings,” says one manufacturer.
Distribute information: Making data accessible makes it useful. “Getting information visibility fostered management’s desire to jump in and improve quality,” claims another.
Keep process consistent: Technology can help enforce best practice processes by providing instruction, reminding of outstanding issues, preventing work from continuing until a step is taken and providing visibility of problems to managers and peers.
Multiple systems deployed
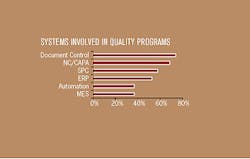
The companies interviewed for this article implement a wide range of different types of systems to improve and support their quality processes, as shown in Figure 2. Most companies have multiple systems that contribute to quality. While the specific issues that arise with these various types of systems can be quite different, some of the key issues are consistent.
Systems must be simple to learn and use, to keep people focused on their primary jobs. Automation systems tend to relieve operators of work. While software systems may take some time to learn and understand, it should be minimal. To have operators prevent quality problems, or at least detect them before final testing or in the lab, operators must be able to use the quality systems quickly and consistently.
Systems must be flexible to accommodate best practices and the ongoing continuous improvement. A top priority for many of these companies in choosing a system was its ability to adapt to their specific needs. Some companies stuck with off-the-shelf technology, while others chose a configurable toolset rather than a fixed application-specific system. And those who could not find a solution to match their process needs built their own system or customized a commercial solution.
Using common systems across many plants can power consistent analysis, and allow rapid roll out of improvements across all facilities. One company says, “Plants all over the country use the same database with the same criteria and codes. This allows us to roll up quality information by plant, product line and division.”
Unfortunately, there is still a major integration gap that is not surprising. Most quality solutions today are oriented on automation and SPC, or are centered on enterprise and plant-wide process and documentation. The two approaches are often entirely separate and not integrated, as shown in the third bar of Figure 3. So the rich process and product data streams available from controls and automation systems are often not fully leveraged. When companies have both, the process data is usually re-keyed into systems that control documentation, non-conformance tracking and corrective action processes.
More sophisticated companies are starting to realize that they have much of the data they need, it’s just not getting fed into their systems. Some are now undertaking projects to integrate the two levels of information from their plant and enterprise quality systems. A few of the most advanced manufacturers have integrated data from programmable logic controllers (PLCs) and control systems into a common data layer that includes data from their quality assurance, ERP and MES systems.
Catch issues early
Why is it so important to capture and analyze process data from automation? For one, because that’s the first possible place to identify problems—or better yet, to spot trends that indicate an emerging problem. This allows companies to take proactive action and prevent quality problems, which in turn saves untold costs in preventing bad product from wasting time, effort and capacity through further value-added processes.
The fact that so much of quality control is still based on testing and inspection of finished goods illustrates that manufacturers have not learned the central message of quality methodologies. This message is to prevent errors and defects, and if they do happen, to catch them as early as possible, not just before shipping. Automation and software systems can often prevent further processing if a parameter is outside of specifications or if a procedure is not followed correctly.
Setting up rigorous processes is not easy, and finding breakthrough solutions to ongoing quality issues requires analysis. Six Sigma strategies can provide a platform for statistical analysis, as can a variety of other methodologies and software systems. One of the challenges some companies face is that much of the data is not easily available for analysis. Data is often stored in documents, not in database format, including data for regulatory compliance, quality documentation, supplier certificates of analysis and product genealogy. This means that organizing data for analysis is a time-consuming and error-prone manual process.
While the task may appear daunting, manufacturers can learn from their peers how to achieve quality and business breakthroughs. The keys to success include culture and management support, adequate resources for projects, clear goal definition, business process changes, focused technology, ongoing training and regular operations audits. And last, but not least, manufacturers must strive for continuous improvements with the diligence to keep the business competitive.
About the Author

Leaders relevant to this article: