Pharma Packaging Machinery Buyers Turn to Automation to Deliver Increased Uptime
For the past decade, the pharmaceuticals industry has gotten a pass on overall equipment effectiveness (OEE). Conventional thinking was that, with margins good, they would focus instead on validation and documentation and not expect OEE to be in the high 80s and low 90s like it was for consumer packaged goods (CPG) manufacturers. Some pharma line efficiency numbers sink lower than 50 percent.
OEE has finally come to pharma, and in a big way. Evidence in a new report, PMMI Business Intelligence, Pharmaceuticals and Medical Devices 2016: Trends and Opportunities in Packaging Operations, shows that pharma machinery buyers are looking for improvements that will drive production and packaging line efficiency, and automation holds the answer.
“Data is needed to monitor machine efficiencies," reported one engineer from a contract manufacturing organization (CMO). "Self -diagnostics is needed."
A process engineer weighed in, “Less operator involved equipment is needed to improve safety, and reduce interaction between operator and machine to increase uptime.”
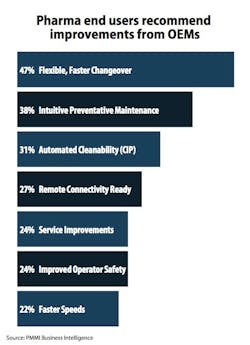
The top five operational improvements driving new equipment purchases include:
- Expanded automation and integration
- Increased throughput
- Ability to measure OEE
- Greater versatility in changeover due to increase in SKUs
- More robotics
Source: PMMI Business Intelligence, Pharmaceuticals and Medical Devices 2016: Trends and Opportunities in Packaging Operations.
Click here for the entire 58-page report: http://www.pmmi.org/Research/ResearchTrends.cfm?ItemNumber=33156