All of the players in the pharmaceutical supply chain are currently challenged to comply with traceability regulations outlined by the U.S. Food & Drug Administration’s (FDA) Drug Supply Chain Security Act (DSCSA). Specifically, last November, the DSCSA required that manufacturers mark item level packages with a unique product identifier, lot number and expiration data. By this November, repackagers must comply with the same serialization mandates, with distributors feeling their first serialization deadline in November of 2019 and dispensers in 2020.
Ultimately, by 2023, there must be full product tracking down to each unit and an interoperable electronic system for traceability across the supply chain. This past February, the FDA issued a new draft guidance around the standards needed for the interoperable exchange of information between supply chain partners.
The goal of the DSCSA is to facilitate the exchange of information at the individual package level about where a drug has been in the supply chain. This enables verification of the legitimacy of the drug product identifier, enhances detection and notification of counterfeit drugs and enables more efficient recalls.
But what’s missing for many organizations is the ability to manage all of this serialization data. This month, Antares Vision, a provider of serialization-based pharmaceutical track and trace technology introduced ATS Four, enterprise-level software that can manage serialization data flow between connecting production plants, contract manufacturers, third-party logistics, healthcare agencies and even government regulators.
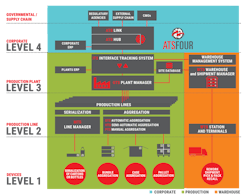
According to the company, as new drug traceability regulations continue to be enforced in the United States, Europe and other key global markets, the need for Level 4 software has become increasingly important. The new ATS Four is built on the Antares Tracking System (ATS) architecture, a track and trace system that has functionality ranging from allocating work orders in ERP to preparing shipments in the warehouse. ATS Four also provides a scalable system that enables connectivity with any traceability framework, establishing an ecosystem that manages the serialization data flow connecting the plants and external entities and agencies.
A key element to managing high volumes of data is an innovative storage system. The ATS Four database technology is engineered to operate at high speed, regardless of the growing amount of data and transactions, the company said.
In addition, ATS Four offers two different installation options: On a cloud platform or on-premises in the local server infrastructure of the pharmaceutical company. A unique capability of the software is that it can be configured on an entirely dedicated cloud space that is not shared with other companies.
Currently, a number of companies around the world have already implemented ATS Four, including Recordati, Salf, Sit, Fine Foods, Marlex, biomo pharma, Renata, Faes, Saneca, Sovereign, Adhex, Sifi, Dms, Sirton, Promed and Falorni. These companies recognize that the software is a comprehensive solution that allows pharmaceutical companies to manage information between the level 1 devices to level 2 production lines to the level 3 plant to the corporate enterprise at level 4 and finally the reporting to regulatory authorities, which is represented by level 5 (see graphic above).
The versatile ATS Four creates a unique point of connection in that it can interface with other brand owners operating with a variety of software and hardware solutions. "We believe our ATS Four enterprise-level serialization software solution is the best on the market,” said Andrew Pietrangelo, president of Antares Vision, North America. “And it represents a major step forward toward helping companies comply with increasingly stringent serialization regulations.”
About the Author
Stephanie Neil
Editor-in-Chief, OEM Magazine

Leaders relevant to this article: