Mobile-Optimized Continuous Monitoring for Pharma Applications
March 15, 2012
2 min read
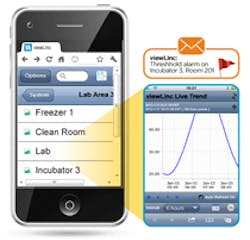
Aimed at highly demanding controlled and regulated environments in the pharmaceutical and biotechnology industries, a new version of continuous monitoring software from Vaisala (www.vaisala.com) gives users an optimized mobile interface for remote monitoring and alarm management. This, along with the location-based reporting and data logging capability, allows users to act on alarms immediately, from anywhere via their smart phones, to improve product safety and ensure compliance with Good Manufacturing Practices and other regulations.
Other key features of Vaisala viewLinc 4.0 include a greatly enhanced user interface that is easier to navigate, more customization capabilities in historical reports, and a live multiple-channel trend display for enhanced reporting. With the new version, users can create reports more quickly in preparation for and during field quality audits. Brad Bayette, tissue services manager at U.S.-based tissue bank said, "We are ecstatic with the Vaisala viewLinc Monitoring System! It not only helps us meet FDA regulatory and accreditation requirements, but we can now monitor multiple sites from any location."
The improved user interface facilitates easy access to product data in a more familiar MS Windows-type navigation. In addition, it shows data by location, which allows users to easily find their loggers, swap out devices, and report data by location. With the mobile interface, users can acknowledge and pause alarms, view live trends on any monitored location under their control, and view trend data in real-time.
Jon Aldous, product manager for Vaisala's Life Science business says, "Like its predecessors, Vaisala viewLinc 4.0 is easily deployed for monitoring temperature, relative humidity, CO2, differential pressure, level, door switches, and more. But, we built this version of viewLinc based on working closely with viewLinc users. Every new feature and interface capability came from feedback and suggestions we got. viewLinc 4.0 addresses the changing needs of our customers and integrates new technologies and user expectations, such as drag and drop and mobile usage."
Language localized versions will be available in May 2012 in French, Chinese, German and Japanese, Aldous added. The company's website includes resources for pharmaceutical manufacturers, including a whitepaper,"Nine Things You Need to Know About Monitoring FDA-regulated Environments" and "50+ FDA Acronyms."
About the Author
Renee Bassett
Managing Editor
Sign up for our eNewsletters
Get the latest news and updates

Leaders relevant to this article: