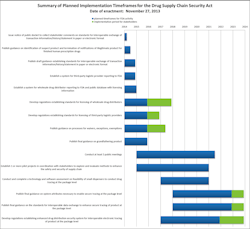
There’s a long list of deadlines associated with the Food and Drug Administration’s (FDA) Drug Supply Chain Security Act (DSCSA), all of which relate to the exchange of transaction information, transaction history, and transaction statements (product tracing information). While some requirements began in November 2014 and several key requirements began at various stages in 2015, the new requirements—as well as the development of standards and the system for product tracing—will continue to be phased in over the next eight years.
In some cases, the FDA has extended policies to provide pharmaceutical companies with more time to become compliant. Regardless of the timeframe, companies must have a plan in place now.
To help in that effort, Avid Solutions, a systems integrator, has partnered with Adents, a provider of serialization and track-and-trace software for the pharmaceutical, cosmetics, and food and beverage industries.
This deal enables Avid Solutions, which specializes in automation, Industrial IT and smart manufacturing technologies, to become a supplier of Adents’ serialization software.
“This partnership allows Avid Solutions to offer an effective, comprehensive serialization solution for our customers, mainly in life sciences and pharmaceuticals, but also in food and beverage and consumer packaged goods,” said Eric Mayer, business development manager at Avid Solutions Inc. “This solution can also be rapidly implemented to achieve FDA compliance per the Drug Supply Chain Security Act.”
With Adents’ Pharma Suite serialization software, companies can accelerate the path to compliance while keeping on top of consumer safety and gaining market insight through new communication channels. The configurable software operates with existing marking and control devices and interfaces with enterprise resource planning (ERP) and manufacturing execution systems (MES). Modules includes:
- Pharma Exchange, which enables the sharing and receiving of serialization data with third parties, converting the data into standardized formats adapted to the requirements of clients and regulatory agencies.
- Pharma Supervisor, deployed on a central server at the site level, enables central configuration of different production lines while managing unit-level serialization and aggregation operations on one or multiple lines simultaneously.
- Pharma Pilot is deployed on each production line to control the marking and vision systems, and manage communication with line equipment to deliver item level serialization and item-bundle-case-pallet aggregation.
“Avid Solutions is a valued, fully trained partner ready to deploy Adents serialization solutions helping life science companies comply with the fast approaching DSCSA deadline in 2017 in a more efficient and cost effective approach,” said Adents Americas vice president James Cummings. “By using our software tools and global track and trace experience, Avid Solutions has increased the company’s safety and security implementation capabilities.”
About the Author
Stephanie Neil
Editor-in-Chief, OEM Magazine

Leaders relevant to this article: